Want to know more about our services? Call, mail, or send us a message.
On 1 August 2025, the International Council for Harmonisation (ICH) has released the draft Q3E Guideline on Extractables and Leachables (E&L) for public consultation. This marks a significant milestone in the global harmonization of impurity control, specifically addressing E&Ls in drug products and drug-device combination products. Building on existing ICH guidance (i.e. Q3A–Q3D (impurities), M7 (mutagenic impurities), and Q9 (quality risk management)), Q3E provides a comprehensive framework for assessing and controlling E&L risks across the entire product lifecycle. A visual overview of this framework is shown in Figure 1, illustrating the iterative nature.

Following the principles outlined in the ICH Q3E framework, extractables and leachables (E&L) studies are tailored to the risk profile of the drug product. This ensures that testing is both scientifically justified and tailored to the product’s intended use.
Safety aspects:
Pharmaceutical quality aspects:
Study designs:
A central concept in E&L risk assessment is the correlation between extractables and leachables
Why correlation matters:
This correlation must be re-evaluated throughout the product lifecycle, especially when changes occur in formulation, packaging, or manufacturing.
After E&L studies, organic leachables exceeding the analytical evaluation threshold (AET) should be identified, quantified, and selected for toxicological risk assessment.
The AET is calculated based on the Safety Concern Threshold (SCT), which is a scientifically derived exposure limit below which a leachable is considered to pose negligible risk for both for mutagenic and non-mutagenic effects.
The SCT is a route- and duration-specific threshold that incorporates the following toxicological principles:
These thresholds were derived from a review of approximately 330 potential leachable PDEs, providing a robust foundation for systemic safety evaluation (Table 1).
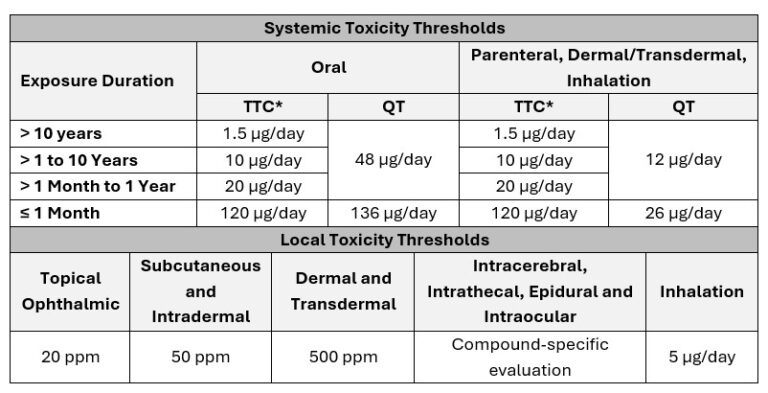
*Note: For ICH S9 (anti-cancer) products, the TTC is not applicable; the SCT is defined by the QT.
Only compounds exceeding the AET are selected for toxicological qualification. This ensures a focused safety evaluation, avoiding unnecessary qualification of compounds with low toxicological relevance.
A key innovation in the ICH Q3E guideline is the emphasis on lifecycle-based re-evaluation of the leachables profile. ICH Q3E acknowledges that leachables may evolve over time due to a variety of factors, and therefore should not be assessed as a one-time exercise. Instead, Q3E promotes ongoing risk management throughout the product’s lifecycle.
ICH Q3E introduces a classification system for leachables (Table 2). This system helps prioritize which compounds require in-depth evaluation and should be revisited during lifecycle-based re-evaluation, especially when new toxicological data or product changes arise.
Class |
Type |
Criteria |
Guidance |
|---|---|---|---|
|
Class 1 – Leachables to be avoided |
(Predicted) Mutagens/Non-Mutagens |
|
Avoid when feasible. Exposure must not exceed compound-specific acceptable exposure level |
|
Class 2 – Leachables to be limited |
(Predicted) Mutagens/Non-Mutagens |
|
Qualified up to the (less-than-lifetime) TTC or QT relevant to the drug product |
|
Class 3 – Leachables with low toxic potential |
Non-mutagens |
chronic PDE in excess of the levels at which leachables are typically observed |
Qualified up to 1.0 mg/day or compound-specific PDE without further safety justification |
AI = acceptable intake ; PDE = permitted daily exposure; QT = qualification threshold; TTC = threshold of toxicological concern
When a leachable exceeds the AET and is selected for qualification, a compound-specific toxicological risk assessment is performed. This process integrates:
· Available toxicological data, including:
o Pharmacological/biological data
o Toxicokinetics
o Systemic toxicity
o Sensitisation potential and local irritation,
o Developmental and reproductive toxicity (DART)
o Genotoxicity and carcinogenicity
o Additional information
· Structure–activity relationships (SAR) and in silico predictions.
· Read-across approaches, using data from structurally/metabolically similar compounds.
The assessment should follow a weight-of-evidence approach, integrating all available data.
To establish a PDE, a Point of Departure (PoD) is selected from the most sensitive and relevant toxicological endpoint, such as
To account for uncertainties in the data and extrapolation across species and populations, uncertainty factors (UFs) are applied. These factors help ensure that the derived PDE is protective of all patient groups, even in the presence of data gaps or variability. While F1–F5 uncertainty factors were already covered in ICH Q3C, Q3E expands the scope by introducing:
These additional factors support the derivation of robust, scientifically justified PDEs, tailored to the product’s route of administration, duration of use, and patient population.
The ICH Q3E framework offers a clear, iterative roadmap for managing extractables and leachables throughout the product lifecycle. By combining risk-based study design, toxicological classification, and ongoing re-evaluation It prioritizes patient safety in all aspects of impurity control.